Audiences
Device Manufacturers
Bridging the gap: MediReport connects device manufacturers to real-world data for enhanced compliance and reliable innovation.

The medical device sector is one of the most highly regulated markets, making device performance data essential for product quality, compliance, and patient safety.
But accessing timely, precise, and reliable data that spans the entire lifecycle can be challenging without a direct line of site into medical operations.
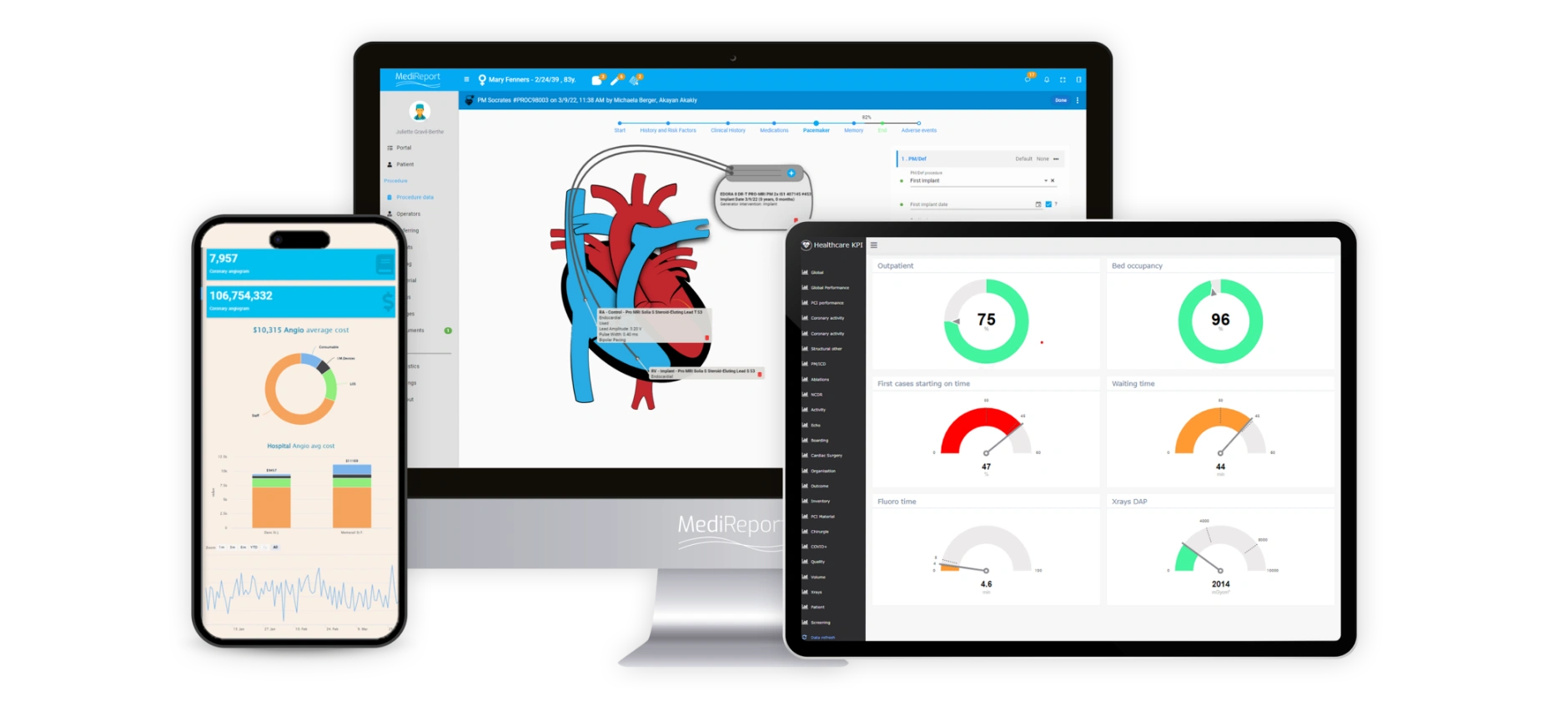
MediReport serves as the bridge between device manufacturers and hospitals, enabling device OEMs to securely gain access to data about the real usage of their devices in real medical settings. This data can be simultaneously utilized to innovate faster, strengthen product reliability and performance, and affirm the highest standards of safety and compliance. We offer support in evaluating the long-term safety and effectiveness of products after they have been approved or introduced to the market, helping identify any potential risks or side effects through post-market surveillance studies.
We believe that real-world data will shape more innovative, capable, and transformative medical equipment and technologies. Through our powerful platform and collaborative partnership, we are committed to bringing these insights to life for medical device manufacturers in a way that decisively answers the big questions while protecting the privacy of hospitals and their patients.

What’s in it for me?
Together with MediReport you can:
Rapidly launch real-world studies and analysis with access to clean, comprehensive, and standardized clinical and administrative data.
Increase the number of participating hospitals for the study by offering clinical teams a single powerful platform to both perform their daily tasks and seamlessly participate in real-world studies without increasing their workload.
Gain multi-site visibility into utilization and performance throughout the device lifecycle with a configurable real-time analytics dashboard.
Ensure the integrity and compliance of your data-driven initiatives with de-identified information that adheres to HIPAA and other applicable regulations.
Automatically capture real-world data from diverse sources including EMR, modalities or information systems without redundant manual entry for clinical teams.
Collect high quality standardized data about the entire medical device lifecycle, granting visibility into the real practices of real cardiologists, clinical support staff, and patients.
Accelerate time-to-market by lowering friction and barriers in medical data collection, compliance, and analysis.
And so much more.
Explore the MediReport 360™ Suite
The MediReport 360™ Suite is a cloud-based solution that seamlessly interfaces with the healthcare environment and intelligently automates workflows while providing data analytics to improve performance and care quality.
 Scheduling
Scheduling
The scheduling module within the MediReport 360™ Suite enables healthcare teams to keep up with their busy workflows effortlessly.
Procedure scheduling is a simple but essential task to properly coordinate staff and allocate rooms and resources. The MediReport 360™ scheduling module brings clinical teams the visibility they need for their day-to-day workflows, as the sophisticated yet easy to use timetable allows them to book appointments, schedule upcoming procedures, check room availability, create physician schedules, and more, all at a glance.
 Structured Reporting
Structured Reporting
Experience effortless reporting with the MediReport 360 Suite Reporting module.
Generate procedure and physician reports in real-time, all from one unified system right at the point of care.
The solution simplifies the reporting process by intuitively guiding clinicians through a structured data entry workflow, ensuring essential procedure data is accurately captured. Being vendor neutral, the MediReport 360 Suite seamlessly interfaces with your facility’s environment, including EMR, Hemodynamic systems, modalities, and PACS, automatically integrating existing data into the report.
Embrace the efficiency of centralized reporting, leading to significant time savings, fewer errors, reduced labor, and enhanced data integrity. To suit the unique needs of every organization, our report templates are fully customizable.
But there’s more. With a structured database forming the backbone of our solution, the MediReport 360 Suite enables immediate repurposing of captured data before, during, and after procedures, without any additional effort or reentry for your clinical team. Data becomes automatically reusable for various initiatives, from CPT coding to reporting, analytics, research, or registry submissions.
 Inventory Management
Inventory Management
Managing inventory across a busy healthcare organization is critical to maintaining operational efficiency. Our flexible web and cloud-based inventory solution automates stock management and device traceability inside the cath lab.
Say goodbye to tedious Excel spreadsheets, confusing paper systems, or eyeballing your stock. The tracer module makes inventory management and device traceability easy and effortless as it delivers real-time visibility over tools and supplies. This solution enables full circle traceability through its efficient system that allows teams to oversee inventory at a glance, optimize resource allocation, reduce costs, and ultimately improve patient care.
 Data Analytics
Data Analytics
MediReport 360™ turns valuable data into actionable information to support evidence-based decision making with a powerful and customizable analytics dashboard that monitors daily activity and tracks clinical, operational, and financial performance across the entire facility, thus improving productivity and quality outcomes.
The solution enables you to report on the most valuable metrics and leverage data for any purpose with high quality clinical and non-clinical data. Capture every last procedure detail and take decisive action quickly, and automatically generate any type of report—including inventory, discharge, pharmacy, procedure, and physician reports—in real time with one click.
 Registries
Registries
MediReport enables healthcare centers to accelerate innovation, safety, and compliance with effortless real-world data capture, as well as easily fulfill their regulatory obligations with one-click registry reporting functionality.
Harness and collect real-world structured data from diverse sources to be leveraged for registry submission, manufacturers’ study participation, real-world analytics, and other initiatives automatically. Access and analyze this data to extend visibility across all of your sites for a complete understanding of organizational, clinical, and financial performance.
 Interoperability / Interfaces
Interoperability / Interfaces
MediReport 360™ easily integrates with the hospital environment, is fully IHE compliant, and is accessible from anywhere at any time through reliable remote access.
MediReport supports flexible interfaces across systems and sites to put an end to lost data, redundancy, and inaccuracy. Data flows seamlessly across the entire landscape, allowing healthcare facilities to realize the unprecedented efficiency, visibility, and understanding that true interoperability allows. Fully cloud-based and securely accessible, MediReport breaks down silos by providing clinical, operational, and financial stakeholders access to the data they need from wherever they are, putting an end to blind spots and information gaps.
