SOLUTIONS
Registries
Accelerating innovation, safety, and compliance with effortless data capture for registry submissions or Real-World Data (RWD) studies.

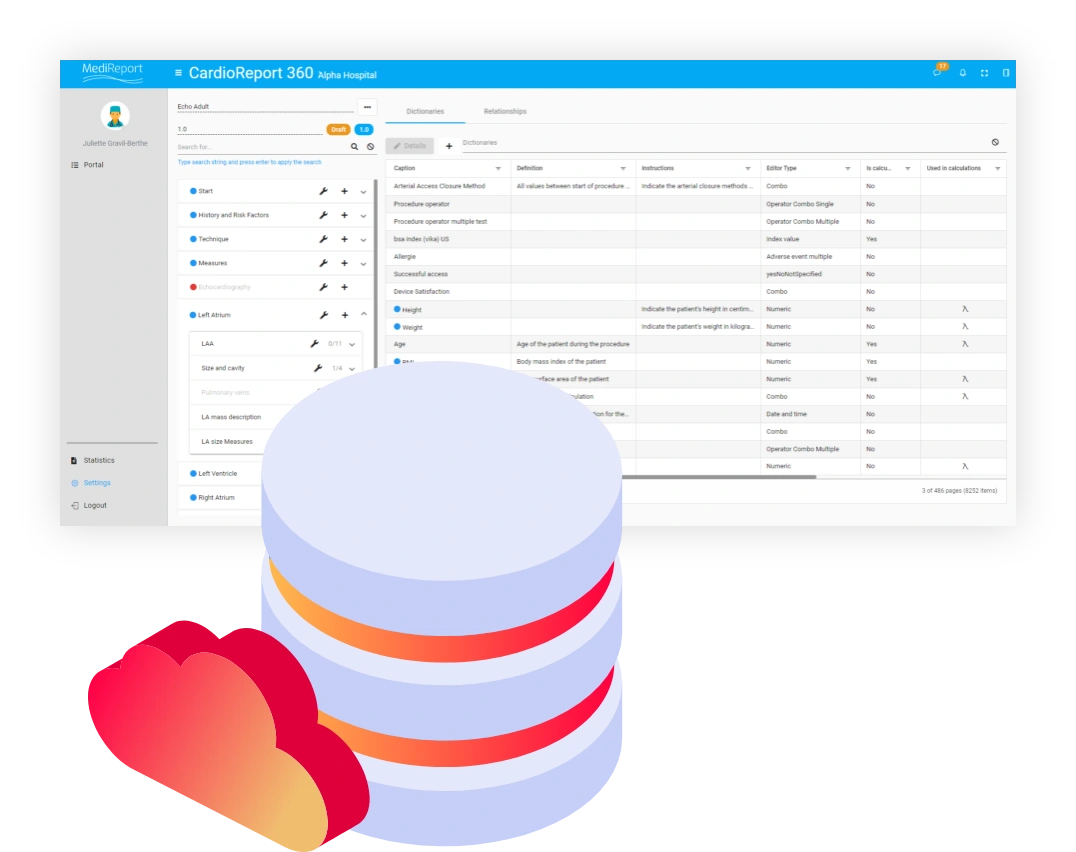
In the complex healthcare ecosystem, there’s a pervasive gap between what stakeholders learn from clinical research and what actually happens in the daily practice.
As a result, stakeholders from clinicians to medical device manufacturers, payers, and medical societies have a growing need for reliable real-world data that reflects clinical realities. But for them, the RWD collection process tends to be tedious and time consuming in an environment where systems are siloed and data is often unstructured. In addition, accessing hospital networks, gaining clinical team buy-in, and implementing the right technology in a short time frame can be challenging.
MediReport serves as a gateway for healthcare professionals to securely gain access to critical data during routine clinical practice and enables the gathering of device usage data in real medical settings.
By connecting technology, real-world insights and people, MediReport enables evidence-based decisions across the healthcare network for better clinical outcomes, increased compliance, and improved patient safety.
What’s possible with RWD empowered by MediReport:
Access real-world, structured data
Easily fulfill regulatory obligations
Enable one-click registry reporting functionality
Submit to registries, manufacturer studies, and more automatically
Leverage data from diverse sources for multiple purposes
Gather data on device usage in medical settings
Experience seamless data submission with direct interfacing
See for Yourself
What’s in it for you?
“MediReport enables healthcare organizations to access real-world, structured data that can be easily leveraged from diverse sources for multiple purposes. This allows them to put the full potential of reporting capabilities into practice with a solution that puts real-world data first.”
— Dr. Serge Makowski, CEO, MediReport
Related Solutions
The MediReport 360™ Suite
A unified platform that integrates workflows and data to reveal efficient insights that transform cardiovascular care.
Inventory Management
A flexible and robust cloud-based inventory solution that automates stock management and device traceability across the cath lab.
Data Analytics
A powerful and customizable real-time data analytics dashboard monitors daily activity and tracks clinical, operational, and financial performance
